Despite lack of FDA approval, Rejuran injections are gaining popularity among U.S. women seeking smoother, more youthful skin.
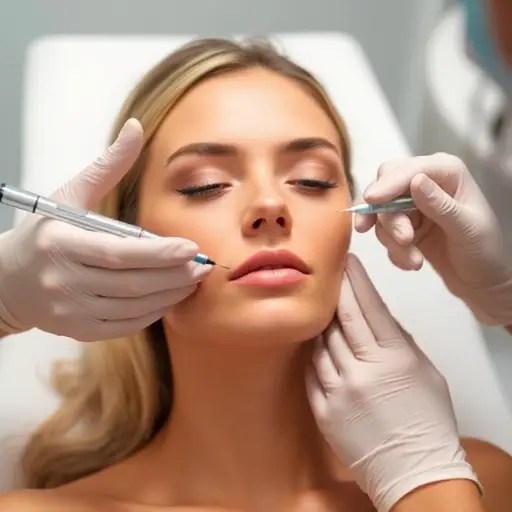
American women are increasingly traveling to South Korea to receive Rejuran, a popular injectable treatment known for its promise of smoother, more youthful skin. Despite not being FDA-approved for injectable use in the U.S., the treatment is available only as a topical serum or cream, and many women are willing to endure the pain and cost to get it injected abroad. Rejuran, which contains DNA fragments derived from salmon cells, is said to hydrate the skin and boost elasticity. It has become a sensation in the K-beauty world, with many women flying to Seoul for sessions that can cost up to $450 per treatment.
Rejuran was first developed in South Korea in 2014 and has since been approved in 20 additional countries for injectable use. Its parent company, PharmaResearch, has seen significant success with the product, and founder Jung Sang-soo has reportedly become a billionaire as a result. However, the company has not confirmed this valuation.
The treatment is administered through a technique known as “serial puncture,” in which hundreds of injections are made near the surface of the skin—typically between 100 to 150 injection points per session. This process is often described as painful, with patients comparing it to “death by 1,000 cuts.” Clinics usually offer topical numbing cream or anesthesia to help manage the discomfort, but the experience remains intense for many. Some patients have described the aftermath as resembling a hive-like rash, which can take up to a few days to subside.
Rejuran is often combined with other treatments, such as collagen-stimulating fillers like Juvelook, ultrasound tightening devices like Ultherapy, and various laser treatments. This combination can make it difficult to isolate the effects of Rejuran alone, as improvements in skin texture and radiance may be attributed to multiple interventions.
Some users report dramatic improvements, including a natural glow, fewer breakouts, and smoother skin. Others, however, see little to no change, and some experience side effects such as irritation, rash, or skin discoloration. Dr. Catherine Chang, a plastic and reconstructive surgeon in Beverly Hills, notes that while anecdotal reports are promising, there is still limited clinical data to confirm Rejuran’s long-term efficacy and safety.
Rejuran’s popularity is also tied to the broader rise of K-beauty, fueled by the global success of K-pop, K-dramas, and social media. This has increased awareness of Korean skincare innovations, making Rejuran a sought-after treatment for women across the world.
While the FDA has not approved Rejuran for injectable use in the U.S., the company has filed an application for approval, and the future of the treatment in the American market remains uncertain. In the U.S., Rejuran is available only as a topical serum or cream, often used in “salmon facial” treatments that combine microneedling or laser resurfacing with the product.
As the demand for Rejuran continues to grow, so does the debate over its safety, effectiveness, and the ethical implications of traveling abroad for unapproved treatments. For now, many women are willing to take the risk, hoping for the radiant, youthful skin they see in their social feeds and the stories of others who have undergone the treatment.



